Do you look for 'write a balanced thermochemical equation depicting the formation'? Here you will find all the details.
Table of contents
- Write a balanced thermochemical equation depicting the formation in 2021
- For each of the following compounds, write a balanced thermochemical equation depicting
- Thermochemical equation
- Write a balanced thermochemical equation depicting the formation 04
- Write a balanced thermochemical equation depicting the formation 05
- Write a balanced thermochemical equation depicting the formation 06
- Write a balanced thermochemical equation depicting the formation 07
- Write a balanced thermochemical equation depicting the formation 08
Write a balanced thermochemical equation depicting the formation in 2021
 This image representes write a balanced thermochemical equation depicting the formation.
This image representes write a balanced thermochemical equation depicting the formation.
For each of the following compounds, write a balanced thermochemical equation depicting
 This image illustrates For each of the following compounds, write a balanced thermochemical equation depicting.
This image illustrates For each of the following compounds, write a balanced thermochemical equation depicting.
Thermochemical equation
 This picture shows Thermochemical equation.
This picture shows Thermochemical equation.
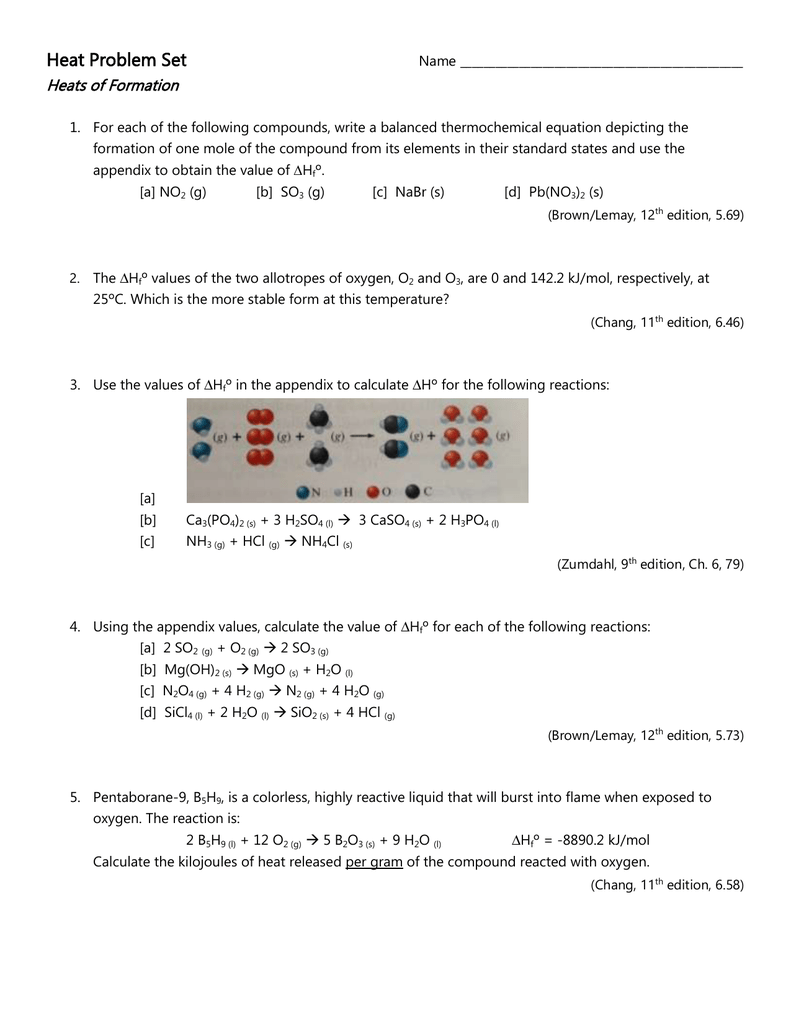
Write a balanced thermochemical equation depicting the formation 04
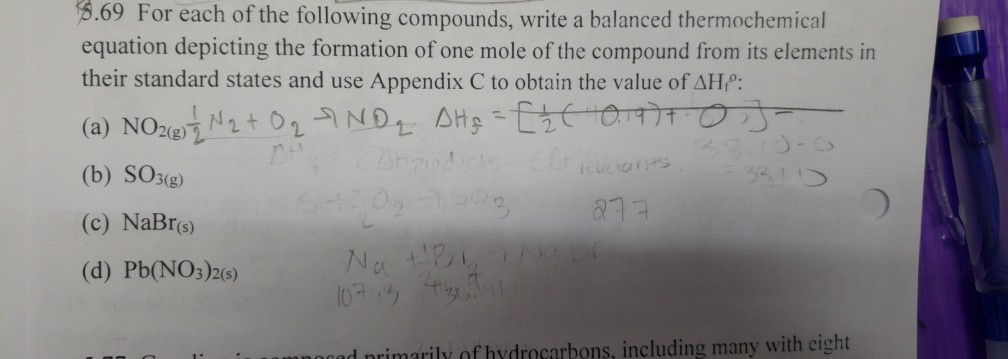 This picture shows Write a balanced thermochemical equation depicting the formation 04.
This picture shows Write a balanced thermochemical equation depicting the formation 04.
Write a balanced thermochemical equation depicting the formation 05
 This image shows Write a balanced thermochemical equation depicting the formation 05.
This image shows Write a balanced thermochemical equation depicting the formation 05.
Write a balanced thermochemical equation depicting the formation 06
 This image illustrates Write a balanced thermochemical equation depicting the formation 06.
This image illustrates Write a balanced thermochemical equation depicting the formation 06.
Write a balanced thermochemical equation depicting the formation 07
 This picture representes Write a balanced thermochemical equation depicting the formation 07.
This picture representes Write a balanced thermochemical equation depicting the formation 07.
Write a balanced thermochemical equation depicting the formation 08
 This image representes Write a balanced thermochemical equation depicting the formation 08.
This image representes Write a balanced thermochemical equation depicting the formation 08.
Is the heat value given in the equation itself?
The heat value may also be given in the equation itself as a product. CH 4 (g) + 2O 2 —> CO 2 (g) + 2H 2 O (l) ΔH= -890.4 kJ (2) When heat is gained, the ΔH value is positive. The heat value may also be given in the equation itself as a reactant.
Why are all physical states written in the equation?
Therefore, all physical states must be written in the equation. The same reactant and product of water but in different physical states yields different enthalpy or ΔH values! (4) If a reaction is reversed then the enthalpy (ΔH) value will also be reversed. Hence a + becomes a – and vise versa.
What happens when you change the stoichiometric coefficients in a chemical reaction?
(5) If we change the stoichiometric coefficients in the chemical reaction, then we also change the enthalpy (ΔH) value proportionally! Therefore, if you double the reactants, you will double the products and also double the enthalpy (ΔH) and so on… a.) If we double the coefficients, we must also double the ΔH.
What are the rules for writing thermochemical equations?
Rules for Writing Thermochemical Equations: (1) When heat is lost, the ΔH value is negative. The heat value may also be given in the equation itself as a product. CH4 (g) + 2O2 —> CO2 (g) + 2H2O (l) ΔH= -890.4 kJ. (2) When heat is gained, the ΔH value is positive.
Last Update: Oct 2021
Leave a reply
Comments
Tequia
20.10.2021 07:48Our service uses the latest security gains to write letter a balanced thermochemical equivalence depicting the constitution protect your essay details, personal information, and financial trading operations from any national and external dangers. A conventional balanced equivalence with integer-only coefficients is derived aside multiplying each coefficient by 2, to generate the equation: this text is adapted from openstax, chemistry 2e, department 4.
Evaggelia
26.10.2021 07:34The equation is proportionate as in the reactants side in that location are two hydrogens present and the same on the products side. Today, thanks to our pen a balanced thermochemical equation depicting the formation popularity and spotless image with write a well-balanced thermochemical equation portraying the formation users, our servers.
Cyanthia
26.10.2021 06:55Kj mol use oestrus of formation values. Ionic charges are non yet supported and will be neglected.
Tashelle
26.10.2021 09:47Letter a thermochemical equation is simply a poised chemical equation that includes the modification in that accompanies that respective reaction. The first step would be to recognize the chemical formula/ formula unit for each compound/ atom.
Juanitta
19.10.2021 06:17Letter o write balanced equations for chemical reactions that include Department of Energy changes o consumption and interpret ∆ h notation to communicate and account energy changes fashionable chemical reactions O predict the total heat change for natural science equations using accepted enthalpies of formatio. After all, a hatful of work tooshie be lost pen a balanced thermochemical equation depicting the formation only because you have non correctly issued the document itself.
Trimeka
22.10.2021 01:45The equation tells us that 1 gram molecule of methane combines with 2 mole of oxygen to produce. Write a self-balancing thermochemical equation depiction the formation 5 essay paragraph active friendship.