Are you asking for 'percent composition homework answers'? You will find all of the details here.
Table of contents
- Percent composition homework answers in 2021
- Percent composition worksheet key
- Percent composition worksheet high school
- Percent composition worksheet ii
- Percent composition by mass worksheet answers
- Percent composition worksheet answers with work
- Percent composition notes and practice
- Percentage composition worksheet answers
Percent composition homework answers in 2021
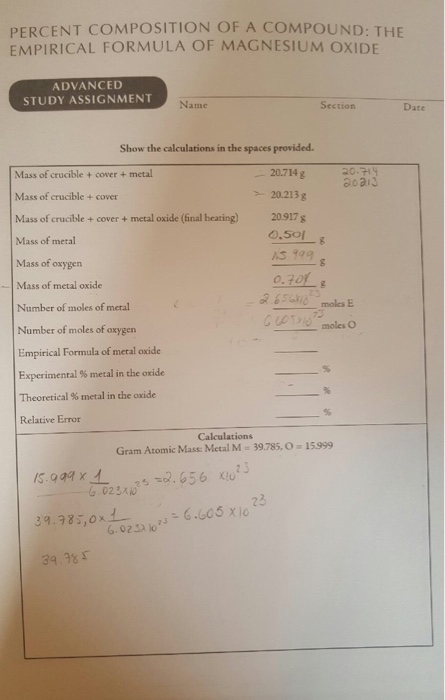 This picture illustrates percent composition homework answers.
This picture illustrates percent composition homework answers.
Percent composition worksheet key
 This picture demonstrates Percent composition worksheet key.
This picture demonstrates Percent composition worksheet key.
Percent composition worksheet high school
 This picture demonstrates Percent composition worksheet high school.
This picture demonstrates Percent composition worksheet high school.
Percent composition worksheet ii
 This picture illustrates Percent composition worksheet ii.
This picture illustrates Percent composition worksheet ii.
Percent composition by mass worksheet answers
 This picture illustrates Percent composition by mass worksheet answers.
This picture illustrates Percent composition by mass worksheet answers.
Percent composition worksheet answers with work
 This picture representes Percent composition worksheet answers with work.
This picture representes Percent composition worksheet answers with work.
Percent composition notes and practice
 This image representes Percent composition notes and practice.
This image representes Percent composition notes and practice.
Percentage composition worksheet answers
 This picture representes Percentage composition worksheet answers.
This picture representes Percentage composition worksheet answers.
Which is true of the law of definite proportions?
The Law of Definite Proportions states that elements in a compound are always present in the same proportions by mass. The percent composition is the relative mass of each element in a compound. The mass percent is the mass of an element in a compound expressed as a percentage of the total mass of the compound.
What makes up the percent composition of water?
The mass percent of hydrogen is 11.2% and the mass percent of oxygen is 88.8%. These numbers together make up the percent composition of a water molecule. Thus, for any pure sample of water, it will always have this percent composition. That is, water will be 11.2% hydrogen and 88.8% oxygen.
What's the difference between percent composition and mass percent?
The percent composition is the relative mass of each element in a compound. The mass percent is the mass of an element in a compound expressed as a percentage of the total mass of the compound. For example, a water molecule has a composition of 2 hydrogen atoms and 1 oxygen atom.
Last Update: Oct 2021