Are you having trouble finding 'olefin ring closing metathesis mechanism'? You will find the answers here.
Ring-closing metathesis ( RCM) is a wide used variation of olefin metathesis stylish organic chemistry for the synthesis of various unsaturated rings via the building block metathesis of 2 terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.Organic Chemistry Portal: RSC ontology ID:
Table of contents
- Olefin ring closing metathesis mechanism in 2021
- Olefin metathesis types
- What is metathesis in chemistry
- Olefin metathesis grubbs
- Ring-closing metathesis pdf
- Cross metathesis mechanism
- Ring-closing metathesis review
- Grubbs catalyst mechanism
Olefin ring closing metathesis mechanism in 2021
 This picture illustrates olefin ring closing metathesis mechanism.
This picture illustrates olefin ring closing metathesis mechanism.
Olefin metathesis types
 This picture representes Olefin metathesis types.
This picture representes Olefin metathesis types.
What is metathesis in chemistry
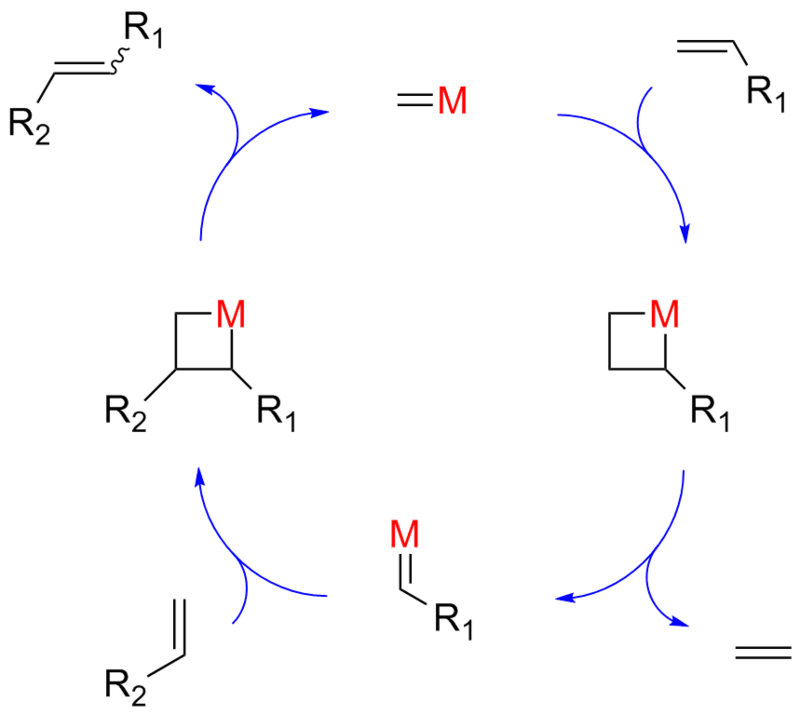 This picture demonstrates What is metathesis in chemistry.
This picture demonstrates What is metathesis in chemistry.
Olefin metathesis grubbs
 This picture shows Olefin metathesis grubbs.
This picture shows Olefin metathesis grubbs.
Ring-closing metathesis pdf
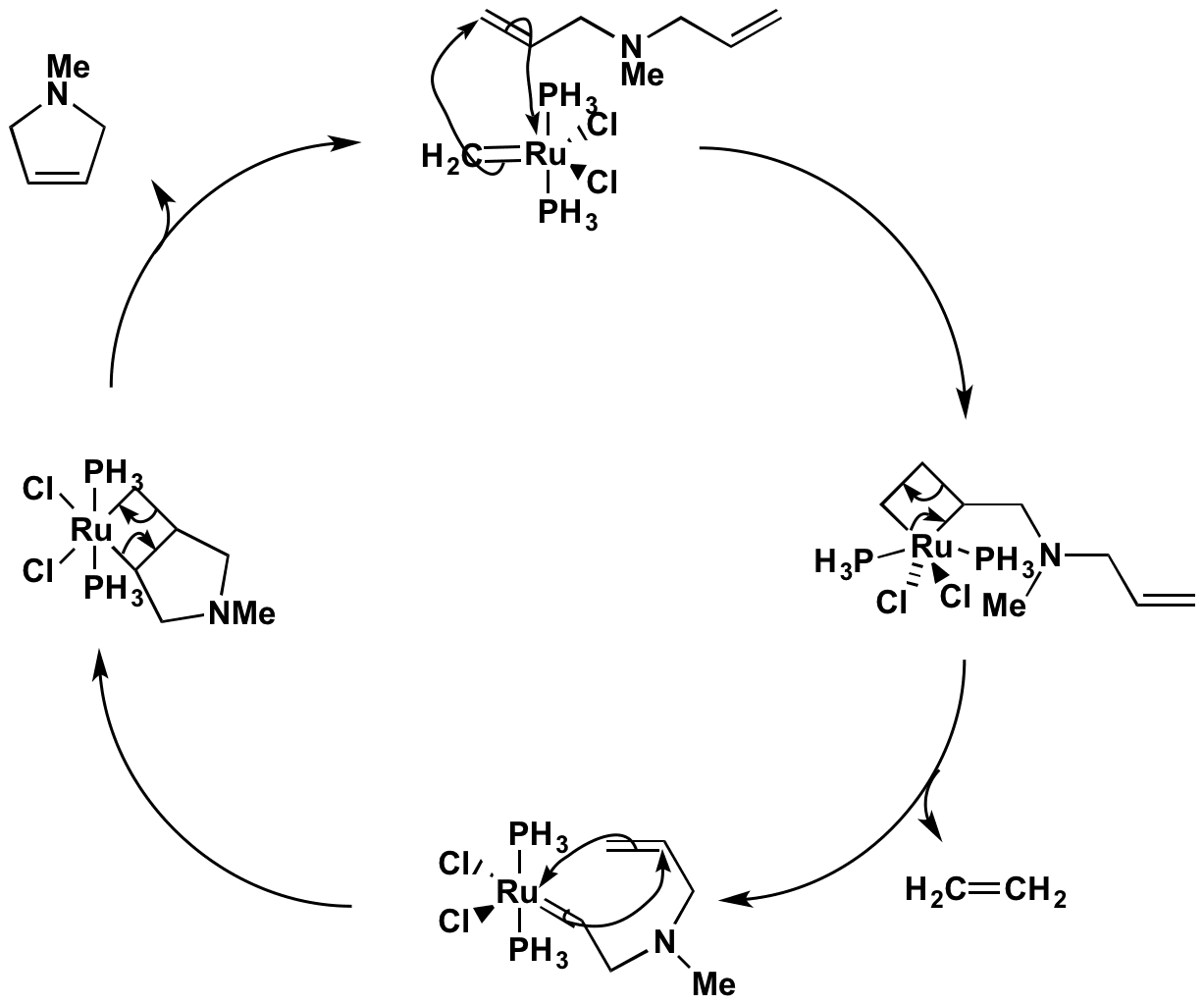 This image shows Ring-closing metathesis pdf.
This image shows Ring-closing metathesis pdf.
Cross metathesis mechanism
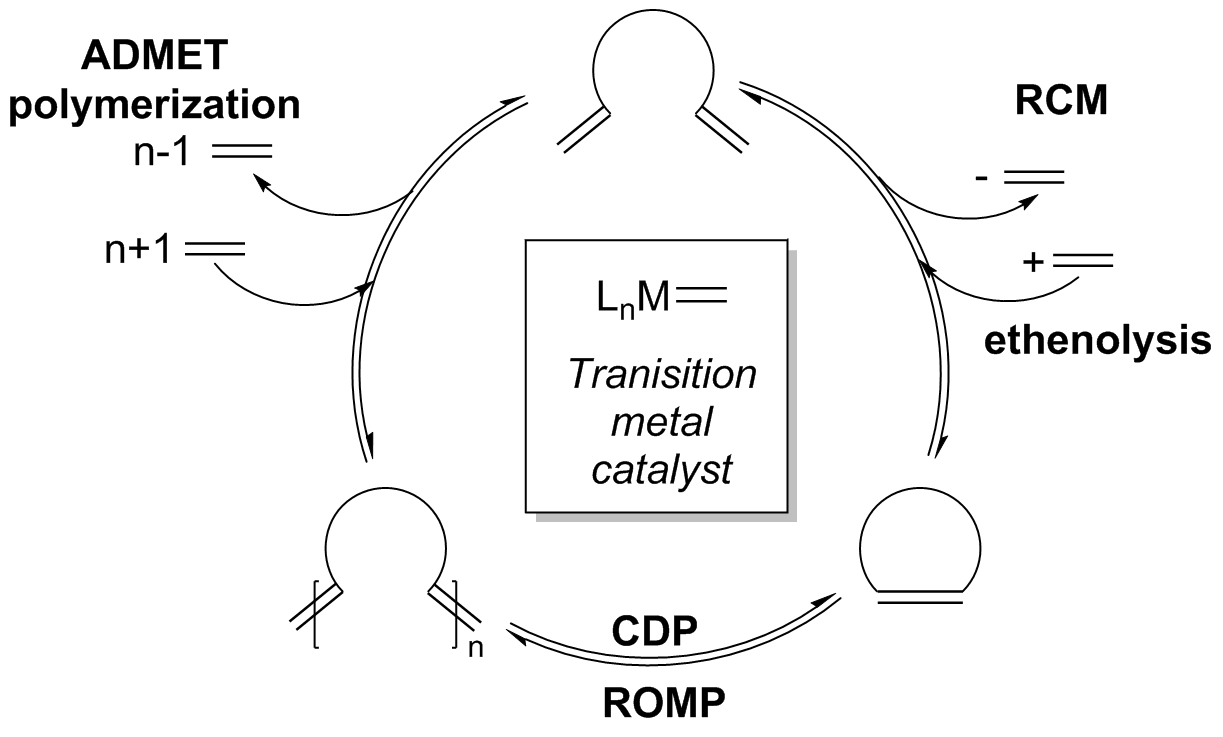 This image illustrates Cross metathesis mechanism.
This image illustrates Cross metathesis mechanism.
Ring-closing metathesis review
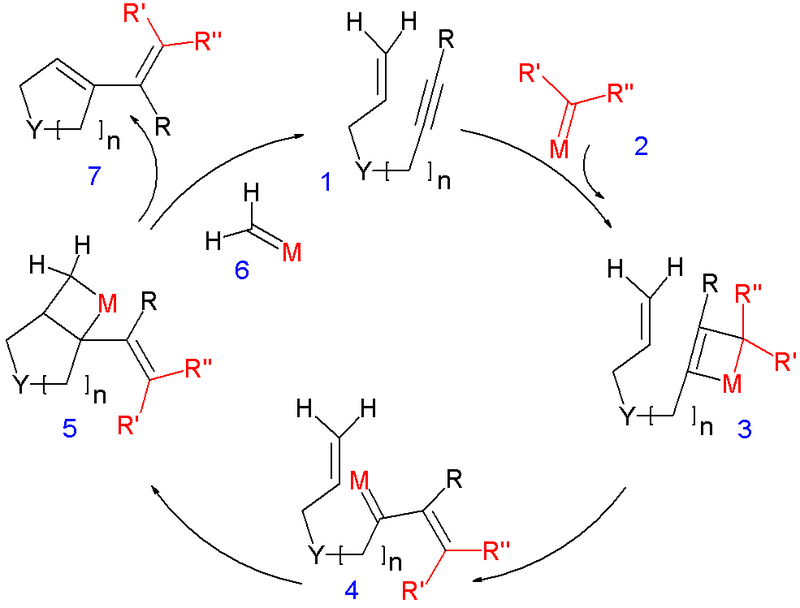 This picture illustrates Ring-closing metathesis review.
This picture illustrates Ring-closing metathesis review.
Grubbs catalyst mechanism
 This image representes Grubbs catalyst mechanism.
This image representes Grubbs catalyst mechanism.
What is the mechanism of ring closing metathesis?
Mechanism of Ring Closing Metathesis. The key intermediate is a metallacyclobutane, which can undergo cycloreversion either towards products or back to starting materials. When the olefins of the substrate are terminal, the driving force for RCM is the removal of ethene from the reaction mixture.
Why is olefin metathesis used in petroleum Reformation?
Olefin Metathesis allows the exchange of substituents between different olefins - a transalkylidenation. This reaction was first used in petroleum reformation for the synthesis of higher olefins (Shell higher olefin process - SHOP), with nickel catalysts under high pressure and high temperatures.
What is the mechanism of olefin metathesis in RCM?
Olefin metathesis is a type of chemical reaction with a wide range of applications. Despite intense study, the mechanism of this reaction and the effects of solvent are still poorly understood. The full RCM catalytic cycle of N-tosyldiallylamine and a Hoveyda–Grubbs catalyst were examined using density functional theory.
How does olefin metathesis work in the Grubbs reaction?
Olefin Metathesis Grubbs Reaction. When molecules with terminal vinyl groups are used, the equilibrium can be driven by the ready removal of the product ethene from the reaction mixture. Ring opening metathesis can employ an excess of a second alkene (for example ethene), but can also be conducted as a homo- or co-polymerization reaction.
Last Update: Oct 2021