Are you interested in finding 'aldol synthesis dibenzalacetone'? You will find the answers here.
The product dibenzalacetone was formed from the reaction between AN acetone molecule and two benzaldehyde molecules. Generally, the aldehyde-alcohol condensation is carried out under A base condition. Atomic number 11 hydroxide was amalgamated with distilled body of water then was victimized to react with sufficient ethanol equally the first dance step.
Table of contents
- Aldol synthesis dibenzalacetone in 2021
- Synthesis of dibenzalacetone side products
- Dibenzalacetone synthesis mechanism
- Preparation of dibenzalacetone
- Synthesis of dibenzalacetone by aldol condensation mechanism
- Synthesis of dibenzalacetone by aldol condensation lab report
- Synthesis of dibenzalacetone from benzaldehyde
- Synthesis of dibenzalacetone lab report
Aldol synthesis dibenzalacetone in 2021
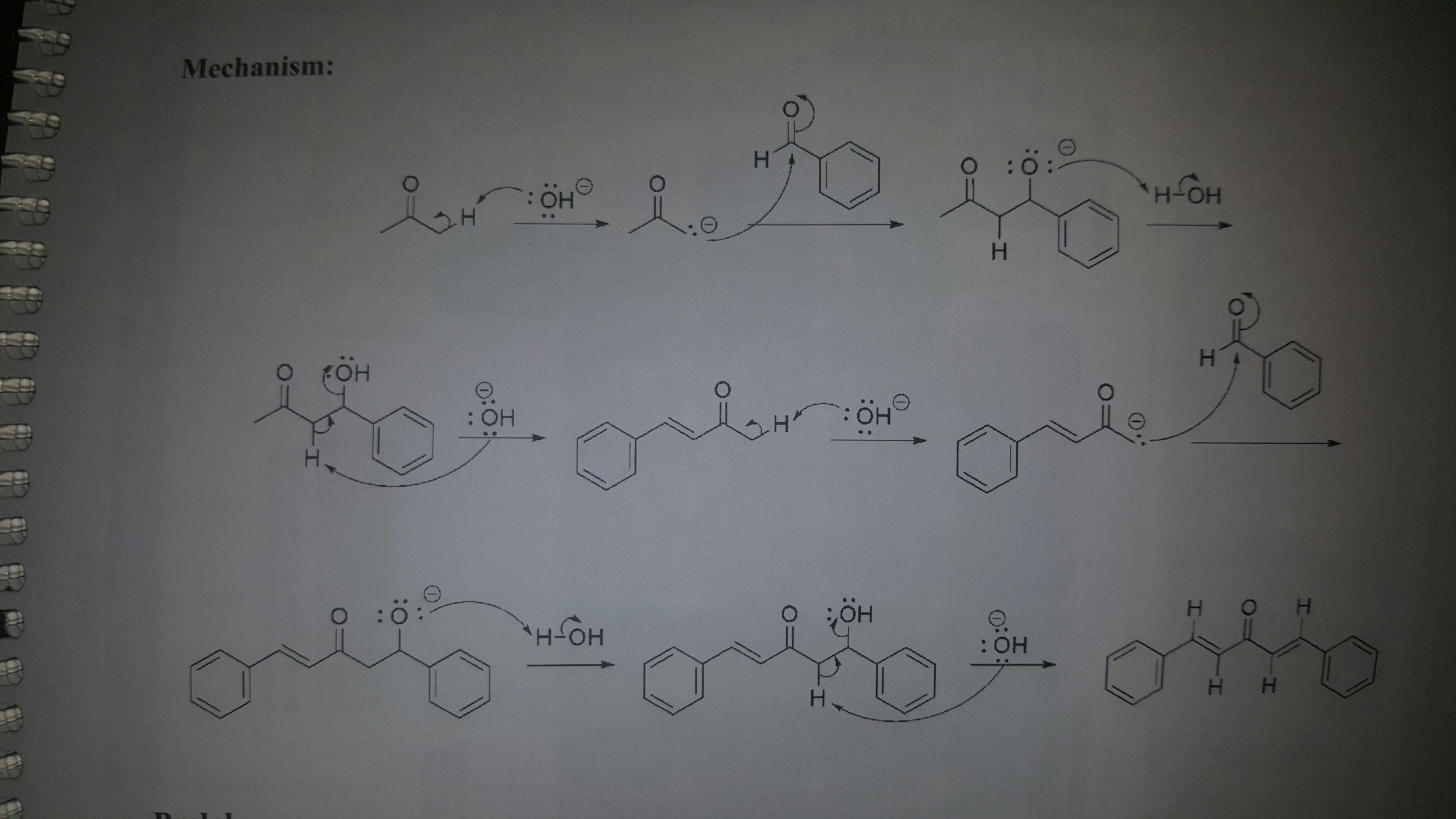 This picture demonstrates aldol synthesis dibenzalacetone.
This picture demonstrates aldol synthesis dibenzalacetone.
Synthesis of dibenzalacetone side products
 This picture shows Synthesis of dibenzalacetone side products.
This picture shows Synthesis of dibenzalacetone side products.
Dibenzalacetone synthesis mechanism
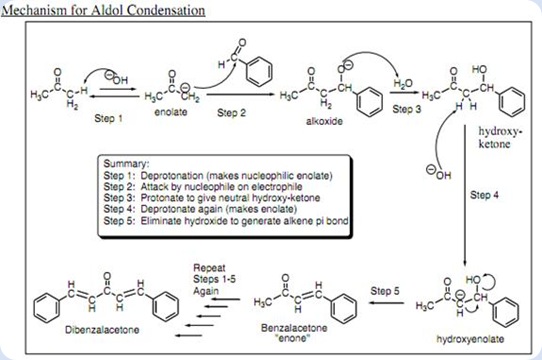 This picture demonstrates Dibenzalacetone synthesis mechanism.
This picture demonstrates Dibenzalacetone synthesis mechanism.
Preparation of dibenzalacetone
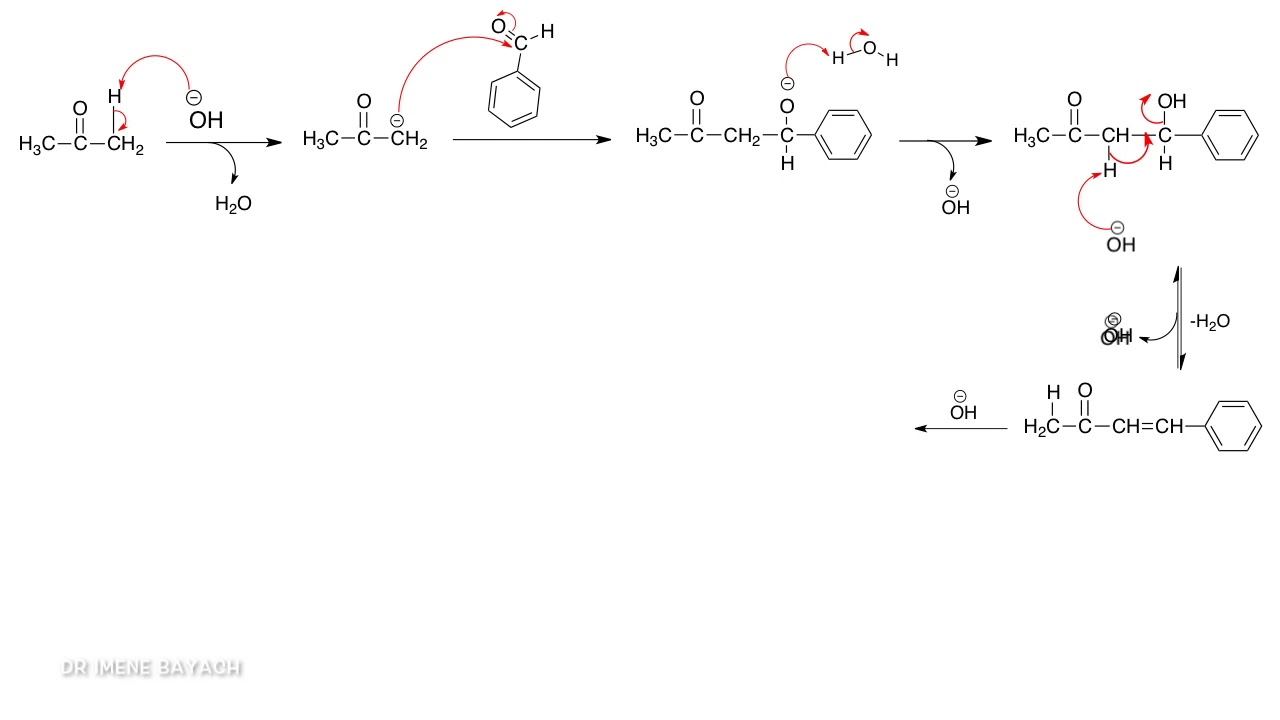 This image shows Preparation of dibenzalacetone.
This image shows Preparation of dibenzalacetone.
Synthesis of dibenzalacetone by aldol condensation mechanism
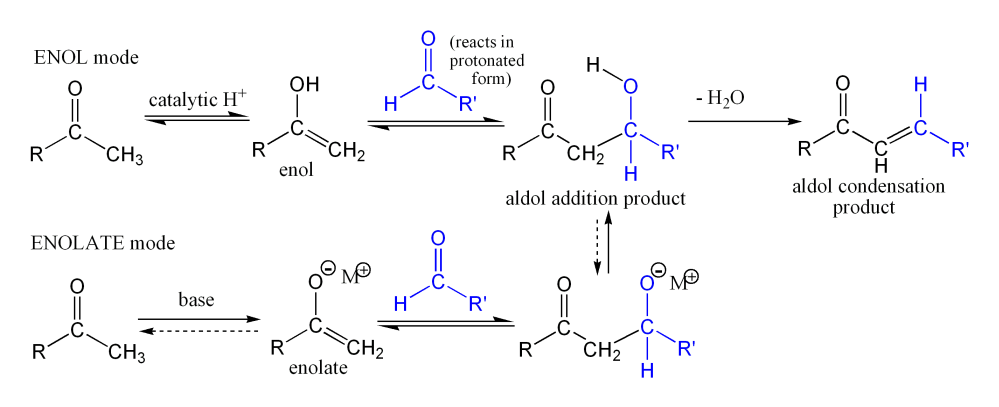 This picture representes Synthesis of dibenzalacetone by aldol condensation mechanism.
This picture representes Synthesis of dibenzalacetone by aldol condensation mechanism.
Synthesis of dibenzalacetone by aldol condensation lab report
 This picture representes Synthesis of dibenzalacetone by aldol condensation lab report.
This picture representes Synthesis of dibenzalacetone by aldol condensation lab report.
Synthesis of dibenzalacetone from benzaldehyde
 This image demonstrates Synthesis of dibenzalacetone from benzaldehyde.
This image demonstrates Synthesis of dibenzalacetone from benzaldehyde.
Synthesis of dibenzalacetone lab report
 This image illustrates Synthesis of dibenzalacetone lab report.
This image illustrates Synthesis of dibenzalacetone lab report.
What is the final weight of dibenzalacetone (1,5-?
The condensation of acetone with the two molecules of benzaldehyde gives dibenzalacetone, otherwise known as 1,5-Diphenyl-1,4-pentadien-3-one. The end product was recrystallized using ethanol. The final weight of my product was .006g.
What is the melting point of dibenzalacetone in recrystallization?
In recrystallization, some of the product dissolved in the ethyl acetate. The melting point of the product is lower than the actual melting point (110 °C ~ 111 °C). This is because there is some impurities exist in the particular compound which will tend to lower the melting point of the dibenzalacetone.
What is the reaction between benzaldehyde and acetone to produce dibenzalacet?
Question 1 page 121 of manual: fConclusion Dibenzalacetone can be synthesized from benzaldehyde and acetone by Aldol condensation. The experiment was fairly successful as the percent yield indicated the experiment was sufficient. The IR graph and the Rf value results also supported the completion of the reaction.
What is an example of an aldol condensation in organic synthesis?
The following equation is an example of an Aldol condensation: Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The Robinson annulation reaction sequence features an aldol condensation; the Wieland-Miescher ketone product is an important starting material for many organic syntheses.
Last Update: Oct 2021